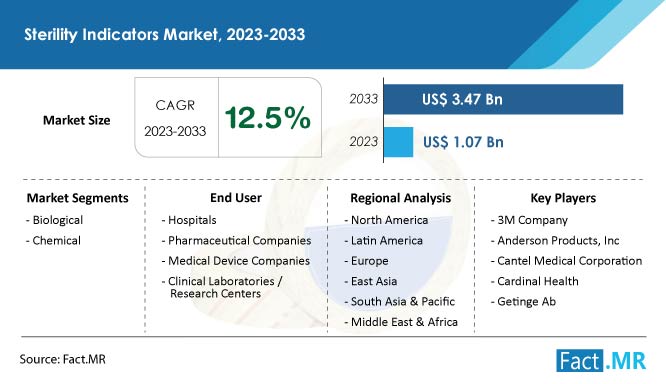
The global sterilization monitoring technologies market is valued at US$ 638.8 million in 2023 and is predicted to reach US$ 1.1 billion by the end of 2033, as per this new industry analysis by Fact.MR, a market research and competitive intelligence provider.
Sterilization technologies are methods used to get rid of all living organisms that can reproduce, such as bacteria, pathogens, infections, fungi, and spores. Sterilization monitoring technologies are widely used by hospitals, pharma and biotech companies, and research institutes. Mechanical, biological, and chemical are three types of sterilization technologies.
Download a Free Sample Copy of this Report:
https://www.factmr.com/connectus/sample?flag=S&rep_id=8391?AS
In biological monitoring, the sterilization process is used to show how it impacts live bacteria that are harder to kill than the majority of the common disease-causing pathogens. Sensitive chemicals are used for chemical monitoring, and these compounds change colour when subjected to high temperatures or temperature-time combinations.
The rising prevalence of hospital-acquired infections and increasing cases related to surgical procedures are some of the factors boosting sales growth. UTIs, pneumonia, meningitis, skin infections, and gastroenteritis are a few examples of infectious diseases that commonly occur in hospitals.
North America currently dominates the global sterilization monitoring technologies market due to the presence of key market players, the rising prevalence of infectious diseases, and the growing rate of the senior population. Europe’s sterilization monitoring technologies market is expected to increase at a healthy pace during the forecast period due to the rapidly developing pharma and biotech industry.
Key Takeaways from Market Study
- Global sales of sterilization monitoring technologies are expected to rise at a CAGR of 6.5% from 2023 to 2033.
- Germany’s market for sterilization monitoring technologies is predicted to expand at a CAGR of 5% during the forecast period.
- Demand for sterilization monitoring technologies in China is expected to rise at an impressive CAGR of 6% over the decade.
- Sales of biological sterilization monitoring technologies are predicted to rise at a high CAGR of 7% through 2033.
“Demand for sterilization monitoring technologies is increasing due to technological advancements in stem cell production and the preservation of bodily cells,” says a Fact.MR analyst.
Recent Market Developments
- STEMart introduced advanced sterility and microbial testing for sterile, non-pyrogenic medical equipment in June 2022.
- In June 2022, Berkshire Sterile Manufacturing unveiled an isolator for sterility testing, which it uses to conduct on-site sterility testing for its GMP samples. The business provides sterile filling of injectable medications, the majority of which are in clinical studies or have a modest commercial demand.
Competitive Landscape
As per this new industry analysis by Fact.MR, a market research and competitive intelligence provider, leading companies in the sterilization monitoring market are adopting several marketing strategies such as new product developments, partnerships, collaborations, regional expansion, local supply, and more.
- Sterigenics upgraded its electron beam facility in Columbia City, Indiana, in May 2022. The Sterigenics Columbia City center offers valuable E-beam sterilization services to protect the safety of medicines and medical devices.
- In July 2020, the 3M unveiled the Attest Mini Auto-reader 490M, a 24-minute internal sterilization monitoring device. Dental sterilization can be facilitated by this technology.
Key Companies Profiled
- 3M Company
- Andersen Products, Inc.
- Bag Health Care GmbH
- Cantel Medical Corporation
- Cardinal Health, Inc.
- Excelsior Scientific
- Getinge AB
- Gke-Gmbh
- Hu-Friedy Mfg. Co., LLC
- Matachana Group
- Mesa Laboratories, Inc.
- PMS Healthcare Technologies Ltd.
- Propper Manufacturing Co., Inc.
- Steris PLC
- Terragene S.A.
Sterilization Monitoring Technologies Industry Research Segmentation
- By Technology :
- Biological
- Chemical
- Mechanical
- By Product :
- Biological Indicators
- Chemical Indicators
- By Method :
- Steam Sterilization
- Ethylene Oxide Sterilization
- Hydrogen Peroxide Sterilization
- Formaldehyde Sterilization
- By Process :
- Pack Monitoring
- Load Monitoring
- Equipment/Process Monitoring
- Exposure Monitoring
- By End User :
- Hospitals
- Pharma & Biotech Companies
- Research & Academic Institutes
More Valuable Insights on Offer
Fact.MR, in its new offering, presents an unbiased analysis of the global sterilization monitoring technologies market, presenting historical demand data (2018 to 2022) and forecast statistics for the period of 2023 to 2033.
The study divulges essential insights on the market based on technology (biological, chemical, mechanical), product (biological indicators, chemical indicators), method (steam sterilization, ethylene oxide sterilization, hydrogen peroxide sterilization, formaldehyde sterilization), process (pack monitoring, load monitoring, equipment/process monitoring, exposure monitoring), and end user (hospitals, pharma & biotech companies, research & academic institutes), across five major regions of the world (North America, Europe, Asia Pacific, Latin America, and MEA).
Check out more related studies published by Fact.MR Research:
https://www.globenewswire.com/news-release/2023/02/14/2607922/0/en/Global-Hernia-Mesh-Devices-Market-Is-Projected-To-Reach-US-6-99-Billion-by-the-End-of-2033-Fact-MR-Analysis.html
Contact:
US Sales Office :
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: sales@factmr.com
